
|
| A carbon molecule ... well, sorta... Photo from www.taylormadeconcepts.co.uk |
Diamonds, as everyone knows, can be very expensive -- just ask any guy thinking of proposing to his future fiance'. However, not many people know that they are made entirely of carbon. Carbon at the earth's surface is normally found as graphite. That's right, the tip of your pencil is made out of the same thing that the sparkly rock on an engagement ring is.
What's the difference? Well, in common terms, the simple answer would be a couple thousand dollars. However, scientifically, graphite and diamonds are very different (yet similar in that they're both made out of just carbon). Graphite has a hardness of 1 on Mohs' hardness scale (from 1 to 10, 10 being the hardest), while a diamond has a hardness of 10 -- the hardest substance known! Mohs' hardness scale is a numerical scale that ranges from 1 (softest) to 10 (hardest). Any given hardness will scratch any mineral with a lower number, so you can try to determine the hardness of any mineral by scratching it against other known minerals. The only thing that can scratch a diamond is another diamond. However, although a diamond is very hard, it is still brittle. Some unethical gem dealers have tried to convince people that if they can smash a diamond with a hammer, the diamond is a fake. This has resulted in these dealers picking up the fragmented pieces and selling them for a profit (Gems and Precious Stones lecture, 2003)! Diamonds also have a very high refractive index and dispersion (one of the reasons why a diamond sparkles so much), and they are a great heat conducter (better than any metal)(Jewelry Plus, 2004). A refractive index is a measure of how light is refracted (or bent) on contact, so the higher the number, the more light it bends; dispersion is the amount of light that a gem disperses. Diamonds show both of these properties in their amazing sparkle. Carbon in graphite is also weakly bonded to one another, while the carbon in diamonds is strongly bonded to four other carbon atoms (Gems and Precious Stones lecture, 2003).
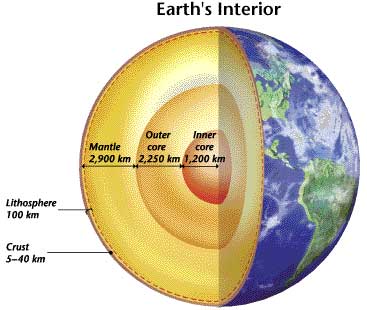
The reason for this difference between graphite and diamonds lies within where a diamond forms -- deep within the earth's mantle (more than 100 miles below). Diamonds need these extra large amounts of pressure and heat to give them their strong bonds. Almost all diamonds are at least one billion years old, and many are over three billion years old. Where diamonds are formed in the mantle, the temperature ranges around 900 to 1,300 degrees centigrade with pressures of 50 to 60 kilobars (the average pressure at the earth's surface is .01 kilobars, while the average temperature is around 15.5 degrees centigrade). (Gems and Precious Stones lecture, 2003). Diamond lovers will be happy to know that we don't have to travel down there to obtain these beautiful diamonds!
|
| Diamonds are from the mantle deep within the earth. This picture is from http://www.phschool.com/at |
|

